
How to Build an FDA-Ready Health App: A Developer’s Guide to Compliance
FDA approval isn’t just a final checkbox — it’s a mindset. In this guide, we break down how to build a compliant health app from the ground up, with practical tips on architecture, documentation, testing, and validation.
Getting FDA approval for a digital health product is more of a marathon than a sprint. One of our clients recently crossed the finish line, with their gamified medical app officially cleared by the FDA after more than two years of reviews and documentation.
This reinforced an important lesson: compliance shouldn’t be an afterthought — it must be embedded from day one.
Before we get into the technical details, here are five key recommendations to help you build a compliant health tech product that’s ready for FDA review.
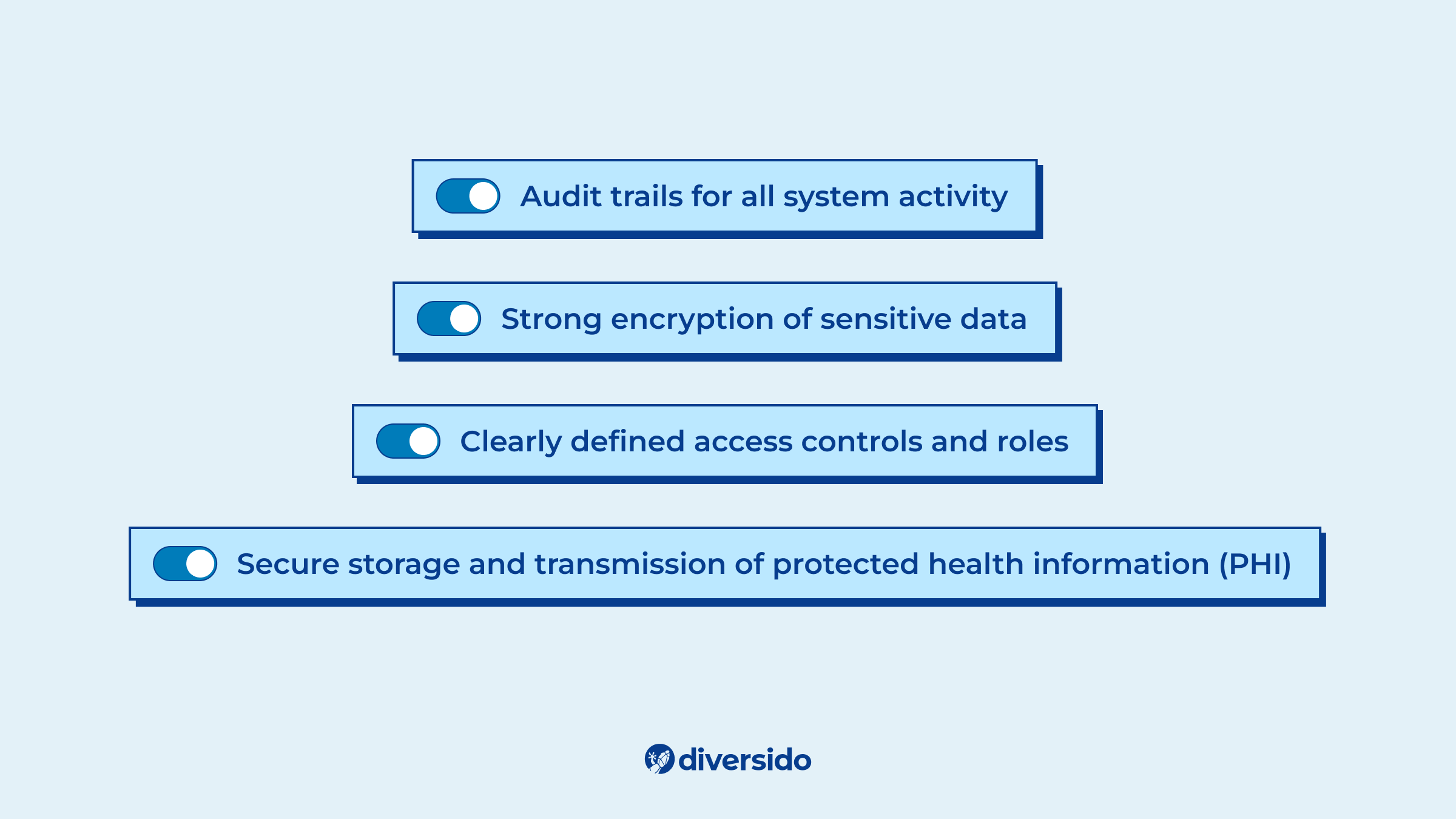
1. Start With Compliance
Make technical decisions early that support a secure and compliant architecture. That means:
- Strong encryption of sensitive data
- Clearly defined access controls and roles
- Audit trails for all system activity
- Secure storage and transmission of protected health information (PHI)
Trying to add compliance later can lead to costly delays. Building with it from the beginning helps you avoid rework and accelerates approval.
2. Document Everything
It’s not enough to show that your product works — the FDA also wants to see exactly how you built and tested it, and how you ensured it’s safe.
- Log every architectural decision, user flow, and release
- Maintain detailed test cases and feature documentation
- Use version control and requirements tracking tools
Good documentation doesn’t just support compliance, it also boosts team efficiency, improves collaboration, and simplifies audits later on.
3. Follow a Controlled Software Development Lifecycle (SDLC)
FDA approval depends on traceability. You’ll need to demonstrate how each feature relates to a specific requirement, and how that requirement was tested and validated.
- Define clear, formalised requirements
- Run risk analyses with mitigation strategies
- Maintain version control (e.g. Git)
- Use traceability matrices to track features and tests
Working in agile cycles is fine — the key is to document the process thoroughly and structure it to align with regulatory expectations.
.png)
4. Test Thoroughly and Validate
Testing isn’t just about proving your app works; it’s about showing how you confirmed that, under various conditions.
- Link each test to a requirement
- Cover edge cases and failure scenarios
- Document your validation methodology and outcomes
FDA reviewers will look at your testing process just as closely as your product. A rigorous, traceable test suite makes approval smoother.
5. Work With a Team That Understands Healthcare
Partnering with a team that understands the regulations and knows how to build with them will save you time, money, and numerous headaches.
At Diversido, we’ve supported digital health companies in meeting FDA, HIPAA, GDPR, and NHS Digital requirements. We know the road to compliance, and we design every system with it baked in from the start.
Final Thoughts
FDA clearance takes work — and that’s how it should be. But with the right structure in place and consistent documentation, it’s absolutely within reach.
Start early. Stay organised. And lean on people who’ve navigated the process before.
Ready to launch your compliant HealthTech app?

FAQ
Do all health apps need FDA approval?
No. Only apps that meet the FDA’s definition of a medical device (meaning they diagnose, treat, prevent, or affect the structure/function of the body) fall under their regulation. Wellness or fitness apps usually don’t need approval, but it's always good to double-check your specific use case.
When should we start thinking about FDA compliance?
From day one. It’s much harder (and more expensive) to retrofit compliance later. If you're building an app that might require FDA review, structure your tech, documentation, and testing processes with that in mind from the start.
Can we use Agile and still be FDA-compliant?
Yes. Agile is allowed — but you’ll need to keep strong documentation. That means clear requirements, version control, test coverage, and traceability from features to test results.
How long does FDA approval usually take?
It depends on the classification of your app and how prepared you are. The process can take anywhere from a few months to over a year. Having a well-documented, validated product can significantly speed things up.
What kind of documentation does the FDA expect?
Expect to submit:
– Your software requirements
– Architecture diagrams
– Risk analyses
– Testing protocols and results
– Validation plans
– Full version history and release notes
It’s not just about your product working, it’s about proving how and why it works safely.
What happens if our app updates frequently?
If your app is FDA-regulated, each update might require re-evaluation depending on the scope. Using a controlled SDLC and documenting each change is key to staying compliant without blocking progress.
Can you help with apps that aren’t FDA-regulated too?
Absolutely. Many best practices for FDA compliance, like secure architecture, clear testing, and strong documentation, are just good product hygiene. We apply them across the board, whether or not the app needs approval.




.webp)

